describe what is happening to the atoms of iron and copper during the reaction
Main Difference – Corrosion vs Oxidation
Corrosion and oxidation are two unlike terms express the same idea. Oxidation reaction is i of the two simultaneous reactions of redox reactions. Corrosion is a type of oxidation. Corrosion happens when the metallic atoms on a metal surface get oxidized in the presence of oxygen and water. The corrosion in iron or steel tin exist recognized every bit rusting. At that place are methods to foreclose metals from rusting. The master departure between corrosion and oxidation is that corrosion happens chiefly on metallic surfaces whereas oxidation tin can happen anywhere.
Central Areas Covered
1. What is Corrosion
– Definition, Explanation of Process, Prevention
2. What is Oxidation
– Definition, Explanation
iii. What is the Relationship Between Corrosion and Oxidation
4. What is the Divergence Between Corrosion and Oxidation
– Comparison of Key Differences
Key Terms: Corrosion, Electroplating, Galvanization, Humidity, Metal, Metal Alloy, Metallic Oxidation, Oxidation, Redox Reaction, Rusting
What is Corrosion
Corrosion is the deterioration of a material due to unlike reactions that take identify on its surface when the material is exposed to the environment. This happens due to the chemical reactions that happen between the surface of the material and the components in air.
Corrosion can occur in both metal and nonmetal surfaces. The corrosion of a fabric affects the structure of the material surface. The well-nigh common example for corrosion is rusting. Here, the color and the quality of the steel is changed. This is acquired by the chemical reaction betwixt the metallic surface and the wet and oxygen in air. Therefore, different varieties of steel have been made in gild to protect the metal from corrosion by irresolute the chemic composition of the metal blend.
Corrosion can also have place in nonmetal surfaces such as tabular array tops and skin. When some corrosive chemicals are dropped on these surfaces, deterioration of that surface may occur. Such chemicals include potent acids and stiff bases.
The corrosion on metals is also known as metallic oxidation. This is because the metal atoms on the surface get oxidized past oxygen in the air in the presence of water. For example, Fe+ii in steel tin can be oxidized to Iron+3 during corrosion of atomic number 26. The rate of corrosion depends on several factors such as the humidity of the air, the surface area of metal that is exposed to air, etc.

Figure i: Corrosion of Iron
In that location are several methods for the prevention of metals from corrosion. Some of these strategies are given below.
- Environmental modifications
- Galvanization – A zinc coating can prevent iron from rusting by interim every bit a sacrificial anode.
- Corrosion inhibitors – These are chemicals that can avoid corrosion by interrupting the oxidation reaction on the metal surface.
- Paints – A coating of paints tin can avoid the initiation of corrosion.
- Electroplating – A sparse layer of a metallic (Ex: Nickel, Chromium) is deposited on steel surface.
Corrosion affects the microstructure of metallic by changing the chemic composition; information technology affects mechanical backdrop and physical appearance of metal.
What is Oxidation
Oxidation is the loss of electrons during a reaction by a molecule, cantlet or ion. Since atoms are equanimous of an equal number of protons and electrons when they are neutral, loss of electrons gives the atoms a positive charge. The degree of oxidation is given equally oxidation country. When an atom undergoes oxidation, the oxidation state of that cantlet increases positively. The opposite of oxidation is reduction.
In that location is always a reduction reaction happening parallel to the oxidation. In other words, an oxidation and a reduction reaction occur simultaneously. These two reactions are called redox reactions. One reaction lonely is called a one-half reaction. The oxidation reaction releases electrons which are and so consumed by the reduction reaction.
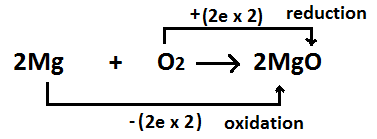
Figure ii: Oxidation of Magnesium
In the by, the oxidation referred to the add-on of molecular oxygen to a compound. But this definition couldn't explain the oxidation reactions that have identify in the absenteeism of molecular oxygen. Compounds that can make other compounds to get oxidized are known every bit oxidizing agents. The species that can get oxidized is called a reducing agent.
Relationship Between Corrosion and Oxidation
- Oxidation is the loss of electrons during a reaction by a molecule, cantlet or ion.
- Corrosion happens when the metal atoms on the surface undergo oxidation.
Deviation Between Corrosion and Oxidation
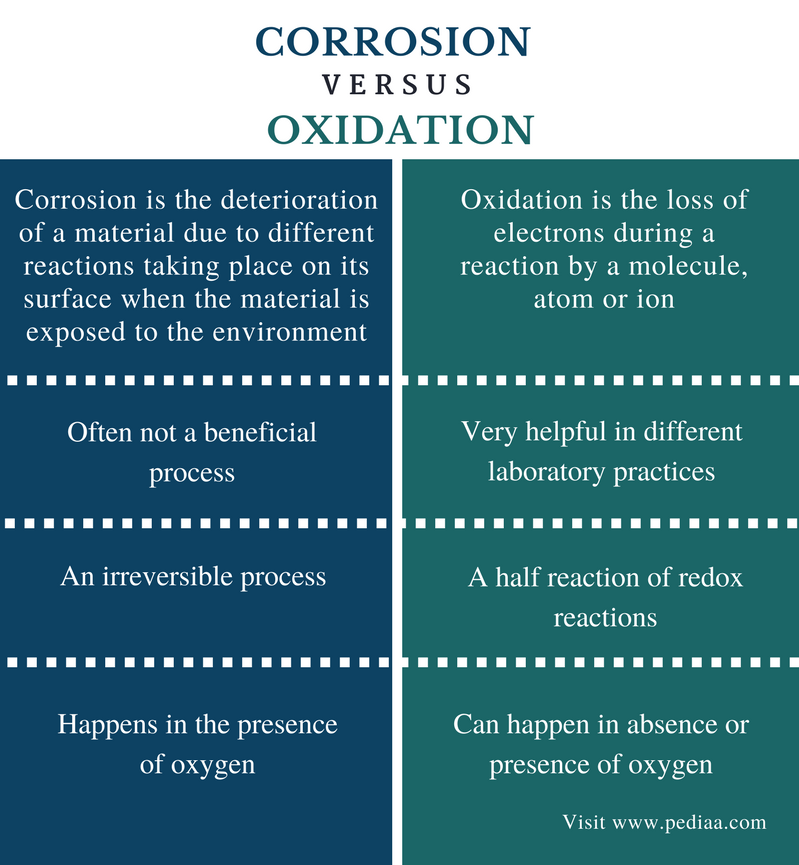
Definition
Corrosion: Corrosion is the deterioration of a textile due to different reactions taking place on its surface when the material is exposed to the environment.
Oxidation: Oxidation is the loss of electrons during a reaction past a molecule, atom or ion.
Benefits
Corrosion:Corrosion is oftentimes not a benign process.
Oxidation: Oxidation reactions are very helpful in different laboratory practices.
Process
Corrosion: Corrosion is an irreversible process.
Oxidation: Oxidation is a one-half reaction of redox reactions.
Oxygen
Corrosion: Corrosion happens in the presence of oxygen.
Oxidation: Oxidation can happen in absence or presence of oxygen.
Conclusion
Corrosion is the deterioration of metallic or nonmetal surfaces. We can recognize rusting of metallic surfaces every bit corrosion. Oxidation reactions always occur with a reduction reaction simultaneously. Oxidation is the loss of electrons from atoms, molecules or ions. The main deviation between corrosion and oxidation is that corrosion happens chiefly on metal surfaces whereas oxidation can happen anywhere.
References:
1. Bell, Terence. "How and Why Do Metals Corrode?" The Residual, Bachelor here.
2. Bell, Terence. "Larn About the Different Methods of Corrosion Prevention for Metals." The Balance, Available hither.
iii. Helmenstine, Anne Marie. "Sympathize What Oxidation Means in Chemistry." ThoughtCo, Available here.
Image Courtesy:
1. "Nandu River Iron Bridge corrosion – 03" By Anna Frodesiak – Own work (CC0) via Commons Wikimedia

Source: https://pediaa.com/difference-between-corrosion-and-oxidation/
0 Response to "describe what is happening to the atoms of iron and copper during the reaction"
إرسال تعليق